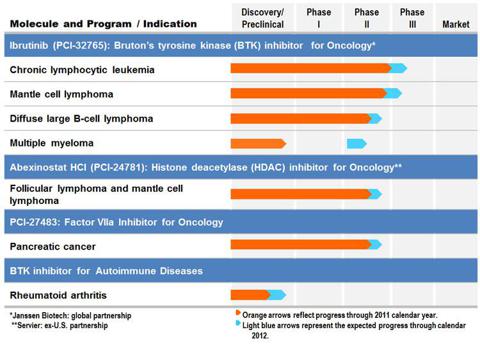
Pharmacyclics Inc. (Nasdaq: PCYC) is a clinical-stage biopharmaceutical company focused on discovering and developing innovative small-molecule drugs for the treatment of cancer and immune mediated diseases. Below is the chart that shows its product pipeline.
| Pharmacyclics Pipeline |
FDA designates Orphan Drug Status for Pharmacyclics Inc's (PCYC) chronic lymphocyctic leukemia treatment.
An orphan drug is a pharmaceutical agent that has been developed specifically to treat a rare medical condition, the condition itself being referred to as an orphan disease which has small market.
Drug result:
Pharmacyclics falls after saying low dose of experimental drug didn't help patients in study
Follow-up Results of Phase 2 Study of Investigational Agent, Ibrutinib, Suggest High and Durable Responses in Relapsed/Refractory Mantle Cell Lymphoma
Drug result:
Pharmacyclics falls after saying low dose of experimental drug didn't help patients in study
Follow-up Results of Phase 2 Study of Investigational Agent, Ibrutinib, Suggest High and Durable Responses in Relapsed/Refractory Mantle Cell Lymphoma
Last Earning:
Revenue for the fiscal quarter ended September 30, 2012 was $102.7 million, compared to $37,000 for the fiscal quarter ended September 30, 2011, an increase of approximately $102.7 million. Revenue for the fiscal quarter ended September 30, 2012 consisted primarily of $100 million of license and milestone revenue due to the Company's achievement of two clinical milestones in connection with the Company's collaboration and license agreement (the "Agreement") with Janssen Biotech, Inc. ("Janssen").
Janssen and Pharmacyclics collaboration
Following regulatory approval, both Pharmacyclics and Janssen will book revenue and co-commercialize ibrutinib. In the US, Pharmacyclics will book sales and take the lead role in US commercial strategy development. Both Pharmacyclics and Janssen will share in commercialization activities. Outside the United States, Janssen will book sales and lead and perform commercialization activities. Profits and losses from the commercialization activities will be split 50/50 on a worldwide basis. Development and commercialization activities under the collaboration will be managed through a shared governance structure. Each company will lead development for specific indications as stipulated in a global development plan, with development costs shared on a 40/60 basis (Pharmacyclics 40% and Janssen 60%).
When will PCYC get its first FDA approval ?
Based on the data, if everything goes right, PCYC will be able to submit its drug for FDA approval late 2013 or early 2014. It takes 6-9 month for FDA to approve any drug. It will be around 2015 when PCYC will be able to generate any revenue from sales and marketing of its first drug.
Why is PCYC trading at 63.25 ?
There are lots of hedge fund manager sitting on this stock based on speculation that something miracle will happen.
What is PCYC performance in last 5 year?
In last 5 year, PCYC is up 61.25 point or 2911%, In one year its up 50.86 point or 410%, year to date its up 48.86 point or 326%, 6 month its up 24 point or 64%.
Conclusion: Based on the valuation, small market target and having no fda approved drug in PCYC profile, 63.25 is way too high.

No comments:
Post a Comment